Zn C2h3o2 2 Molar Mass
scising
Sep 13, 2025 · 5 min read
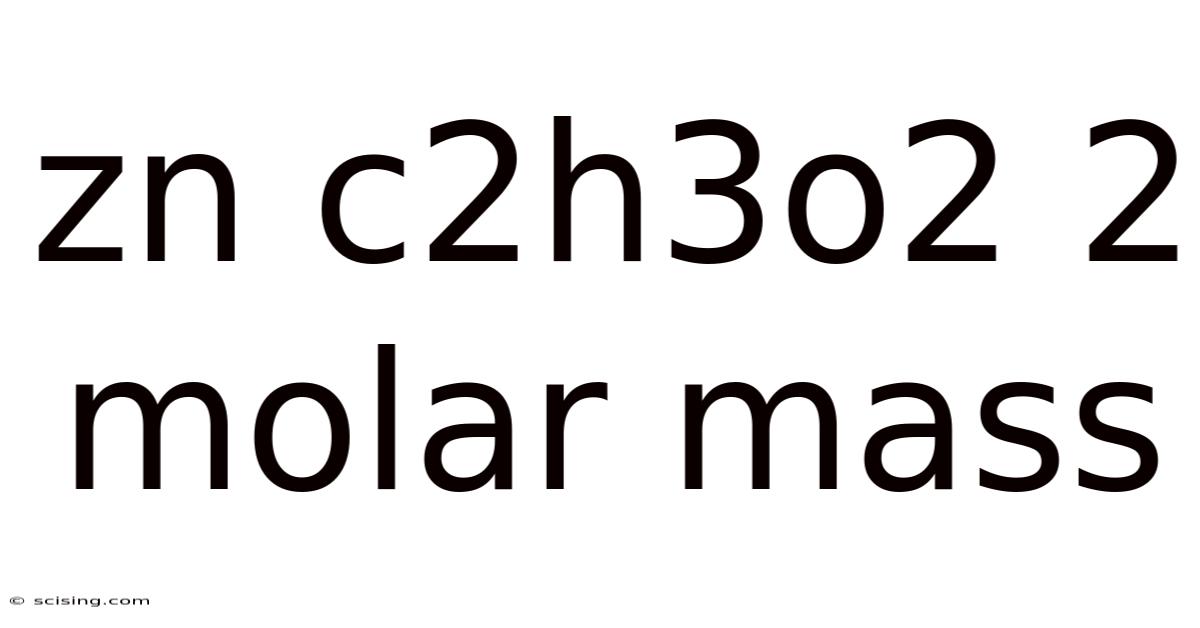
Table of Contents
Unveiling the Molar Mass of Zinc Acetate Dihydrate (Zn(C₂H₃O₂)₂·2H₂O): A Deep Dive
Determining the molar mass of a compound is a fundamental concept in chemistry, crucial for various calculations, from stoichiometry to solution preparation. This article will delve into the detailed calculation of the molar mass of zinc acetate dihydrate, Zn(C₂H₃O₂)₂·2H₂O, often abbreviated as ZnAc₂·2H₂O. We'll break down the process step-by-step, explore the significance of understanding molar mass, and address frequently asked questions. This comprehensive guide aims to equip you with a thorough understanding of this essential chemical concept.
Understanding Molar Mass: The Foundation
Before jumping into the calculation, let's solidify our understanding of molar mass. The molar mass of a substance is the mass of one mole of that substance, expressed in grams per mole (g/mol). One mole is defined as Avogadro's number (approximately 6.022 x 10²³) of constituent particles, whether they are atoms, molecules, or ions. Essentially, the molar mass tells us the mass of a very large, but precisely defined, collection of particles.
To determine the molar mass of a compound, we need to consider the atomic masses of each element present and the number of atoms of each element in the compound's chemical formula. Atomic masses are usually found on the periodic table and are expressed in atomic mass units (amu) or, for our purposes, grams per mole (g/mol).
Calculating the Molar Mass of Zn(C₂H₃O₂)₂·2H₂O
Now, let's meticulously calculate the molar mass of zinc acetate dihydrate, Zn(C₂H₃O₂)₂·2H₂O. This compound contains:
- Zinc (Zn): One zinc atom.
- Carbon (C): Four carbon atoms (2 x 2).
- Hydrogen (H): Six hydrogen atoms (2 x 2 + 2 x 2).
- Oxygen (O): Six oxygen atoms (2 x 2 + 2).
We'll use the following approximate atomic masses from the periodic table:
- Zn: 65.38 g/mol
- C: 12.01 g/mol
- H: 1.01 g/mol
- O: 16.00 g/mol
Here's the step-by-step calculation:
-
Zinc (Zn): 1 atom x 65.38 g/mol = 65.38 g/mol
-
Carbon (C): 4 atoms x 12.01 g/mol = 48.04 g/mol
-
Hydrogen (H): 6 atoms x 1.01 g/mol = 6.06 g/mol
-
Oxygen (O): 6 atoms x 16.00 g/mol = 96.00 g/mol
-
Total Molar Mass: 65.38 g/mol + 48.04 g/mol + 6.06 g/mol + 96.00 g/mol = 215.48 g/mol
Therefore, the molar mass of zinc acetate dihydrate, Zn(C₂H₃O₂)₂·2H₂O, is approximately 215.48 g/mol. It's crucial to note that slight variations might occur depending on the atomic masses used, as these values are typically rounded. Using higher precision atomic mass values will yield a more precise molar mass.
The Significance of Molar Mass Determination
Understanding and accurately calculating molar mass is crucial for various applications in chemistry and related fields:
-
Stoichiometry: Molar mass is essential for performing stoichiometric calculations, which involve determining the quantities of reactants and products in chemical reactions. It allows us to convert between mass and moles, enabling precise predictions of reaction yields.
-
Solution Preparation: When preparing solutions of a specific concentration (e.g., molarity), knowing the molar mass is essential for accurately weighing out the required mass of the solute.
-
Analytical Chemistry: Molar mass plays a vital role in various analytical techniques, such as titration and gravimetric analysis, where the mass of a substance is used to determine its concentration or quantity.
-
Material Science: In material science, accurate molar mass determination is crucial for understanding the properties and behavior of materials, particularly those with complex molecular structures.
-
Pharmaceutical Applications: Precise molar mass calculations are critical in pharmaceutical chemistry for determining the correct dosages of drugs and formulating medications.
Beyond the Basics: Understanding the Dihydrate Nature
The ".2H₂O" in the chemical formula Zn(C₂H₃O₂)₂·2H₂O signifies that two water molecules are associated with each formula unit of zinc acetate. This is referred to as a dihydrate. These water molecules are not chemically bonded to the zinc acetate but are instead held within the crystal lattice structure through weaker intermolecular forces such as hydrogen bonding.
The presence of these water molecules significantly affects the molar mass. If we were calculating the molar mass of anhydrous zinc acetate, Zn(C₂H₃O₂)₂, we would exclude the mass contribution of the two water molecules, resulting in a lower molar mass. The inclusion of the water molecules in the calculation accurately represents the mass of the compound as it exists in its commonly encountered hydrated form.
Frequently Asked Questions (FAQs)
Q1: What happens if I use different atomic masses from the periodic table?
A1: Using slightly different atomic masses from the periodic table, reflecting different levels of isotopic abundance, will lead to a slightly different molar mass. However, the difference will usually be minimal and insignificant for most applications.
Q2: How does the dihydrate affect the properties of zinc acetate?
A2: The presence of water molecules in the dihydrate can influence the solubility, hygroscopicity (ability to absorb moisture), and crystal structure of zinc acetate. Anhydrous zinc acetate may have different properties.
Q3: Can I easily convert between anhydrous and dihydrate forms of zinc acetate?
A3: Yes, often careful heating (dehydration) can remove the water molecules from the dihydrate, converting it to the anhydrous form. The reverse process (hydration) can occur under specific conditions of humidity.
Q4: Where can I find reliable atomic masses for these calculations?
A4: Reliable atomic masses can be found on various online periodic tables, chemistry handbooks, and educational resources. The values are often presented to several decimal places for higher precision.
Q5: Are there other hydrates of zinc acetate besides the dihydrate?
A5: While the dihydrate is the most common form, other hydrates of zinc acetate might exist under specific conditions, although they are less frequently encountered.
Conclusion: Mastering Molar Mass Calculations
Calculating the molar mass of a compound like zinc acetate dihydrate is a foundational skill in chemistry. This process involves understanding chemical formulas, utilizing atomic masses from the periodic table, and performing simple arithmetic. The accurate determination of molar mass is critical for a broad range of chemical calculations and applications, extending far beyond the classroom into diverse scientific and industrial settings. By understanding the steps involved and the significance of the result, you can confidently tackle similar calculations and apply this knowledge to more complex chemical problems. Remember to always double-check your work and use precise atomic masses for the highest accuracy.
Latest Posts
Latest Posts
-
Why Is Chichen Itza Important
Sep 13, 2025
-
Writing Inequalities From Word Problems
Sep 13, 2025
-
Do Exponential Functions Have Asymptotes
Sep 13, 2025
-
Decoding Vs Encoding In Reading
Sep 13, 2025
-
Echoic Memory Ap Psychology Definition
Sep 13, 2025
Related Post
Thank you for visiting our website which covers about Zn C2h3o2 2 Molar Mass . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.