Formula For Iron 2 Oxide
scising
Aug 21, 2025 · 7 min read
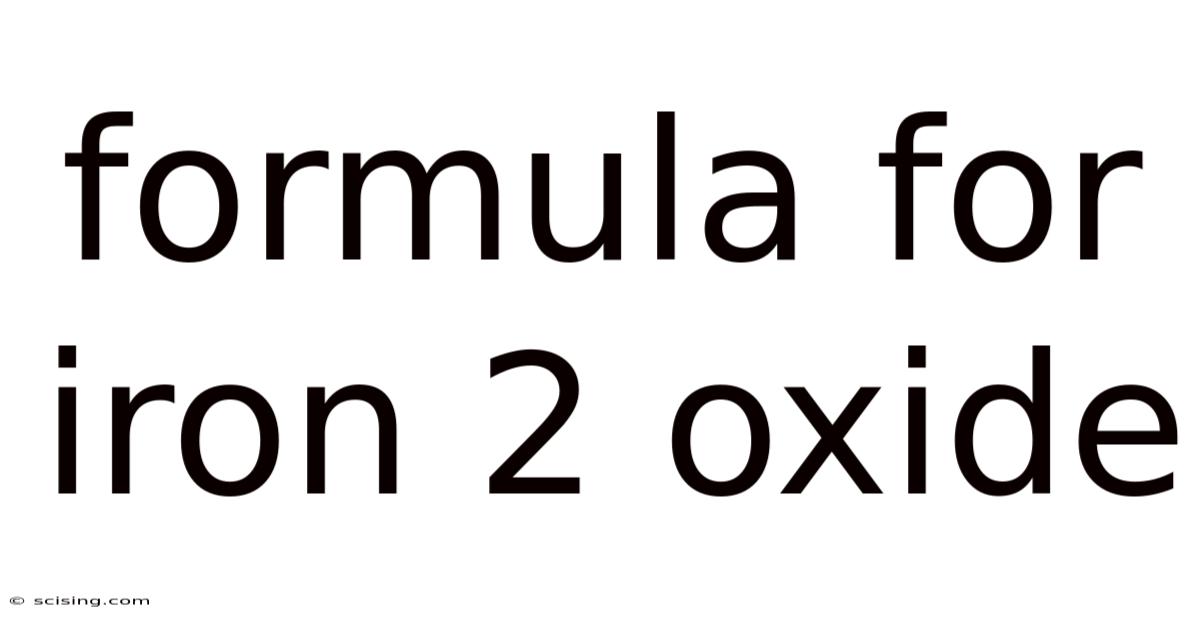
Table of Contents
Unveiling the Formula for Iron(II) Oxide: A Deep Dive into its Properties, Applications, and Significance
Iron(II) oxide, also known as ferrous oxide, is a fascinating chemical compound with a wide array of applications and a significant role in various scientific fields. Understanding its chemical formula, properties, and uses is crucial for anyone studying chemistry, materials science, or related disciplines. This comprehensive article will delve deep into the world of iron(II) oxide, exploring its formula, characteristics, production methods, applications, and safety considerations. We'll unravel the mysteries behind this seemingly simple compound and illuminate its significant contributions to our daily lives.
Understanding the Chemical Formula: FeO
The chemical formula for iron(II) oxide is FeO. This simple notation tells us a great deal about the compound's composition. 'Fe' represents the element iron (Ferrum in Latin), and 'O' represents oxygen. The Roman numeral II, or the prefix "ferrous," indicates that iron is in its +2 oxidation state. This means each iron atom has lost two electrons, resulting in a charge of +2. Oxygen, on the other hand, typically has an oxidation state of -2. The combination of one iron(II) ion (Fe²⁺) and one oxide ion (O²⁻) creates a neutral compound, hence the 1:1 ratio in the formula FeO.
Properties of Iron(II) Oxide: A Closer Look
Iron(II) oxide exhibits several key properties that contribute to its versatility and wide range of applications:
-
Appearance: Pure iron(II) oxide is a black, crystalline solid. However, depending on its preparation method and impurities present, it can appear dark gray or even brownish-black.
-
Crystal Structure: FeO adopts a rock-salt crystal structure, where iron(II) ions and oxide ions are arranged in a face-centered cubic lattice. This structure is relatively stable and contributes to the compound's hardness.
-
Melting Point: Iron(II) oxide has a relatively high melting point, typically around 1377 °C (2511 °F). This high melting point reflects the strong ionic bonds between the iron(II) and oxide ions.
-
Solubility: Iron(II) oxide is insoluble in water but dissolves readily in dilute acids. This property is crucial in several industrial processes.
-
Magnetic Properties: While not a strong magnet itself, iron(II) oxide displays some antiferromagnetic properties below its Néel temperature (approximately 198 K or -75 °C). This means the magnetic moments of neighboring iron ions are aligned antiparallel, resulting in a net magnetic moment of zero.
-
Reactivity: FeO is relatively reactive, readily oxidizing to iron(III) oxide (Fe₂O₃) in the presence of air and moisture. This oxidation process is a significant factor in the degradation of iron-containing materials.
Production Methods: Synthesizing Iron(II) Oxide
Several methods exist for producing iron(II) oxide, each with its advantages and disadvantages:
-
Reduction of Iron(III) Oxide: One common method involves reducing iron(III) oxide (Fe₂O₃) with hydrogen gas (H₂) at high temperatures. This reaction produces iron(II) oxide and water vapor:
Fe₂O₃(s) + H₂(g) → 2FeO(s) + H₂O(g)
This process needs careful control of temperature and pressure to avoid further reduction to metallic iron.
-
Thermal Decomposition of Iron(II) Oxalate: Heating iron(II) oxalate (FeC₂O₄) in an inert atmosphere (e.g., nitrogen gas) leads to the decomposition of the oxalate and the formation of iron(II) oxide:
FeC₂O₄(s) → FeO(s) + CO(g) + CO₂(g)
This method offers a higher level of purity but requires precise temperature control.
-
Direct Reaction of Iron and Oxygen: While theoretically possible, directly reacting iron metal with oxygen to produce solely iron(II) oxide is challenging. The reaction often proceeds to form iron(III) oxide (Fe₂O₃) as the major product.
Applications of Iron(II) Oxide: A Versatile Compound
Iron(II) oxide's unique properties make it a versatile material with a broad range of applications across various industries:
-
Pigments and Ceramics: Iron(II) oxide is used as a pigment in various applications, including ceramics, glass, and paints. It imparts a characteristic dark gray or black color. The color intensity and shade can be modified by controlling the particle size and purity of the oxide.
-
Iron and Steel Industry: Iron(II) oxide plays a vital role in the iron and steel industry. It serves as a precursor in the production of iron and steel, and its presence influences the properties of the final product.
-
Catalysis: Iron(II) oxide acts as a catalyst in several chemical reactions. Its catalytic activity stems from its ability to change its oxidation state readily. It's used in various catalytic processes, including those involved in the production of chemicals and fuels.
-
Magnetic Materials: While not a strong ferromagnet itself, iron(II) oxide can be incorporated into composite materials to enhance their magnetic properties. Research is ongoing in exploring its use in novel magnetic applications.
-
Water Treatment: Iron(II) oxide can be used in water treatment processes to remove certain contaminants. Its reactivity helps in the precipitation and removal of unwanted substances.
-
Powder Metallurgy: Iron(II) oxide is used as a component in powder metallurgy, where metallic powders are compacted and sintered to create complex shapes. The oxide can act as a binder or influence the final properties of the metal part.
-
Geochemical Applications: The study of iron(II) oxide is important in geological studies, where it is found in various minerals and rocks. Its presence and chemical state can provide valuable insights into the formation and evolution of geological formations.
-
Nanotechnology: Iron(II) oxide nanoparticles are currently under investigation for potential applications in various areas, such as medicine (drug delivery), electronics, and energy storage.
Safety Considerations: Handling Iron(II) Oxide Responsibly
While iron(II) oxide is generally considered to be non-toxic, certain safety precautions are essential when handling it:
-
Dust Inhalation: Inhalation of iron(II) oxide dust can irritate the respiratory system. Appropriate respiratory protection, such as dust masks, should be used when handling the powdered form of the compound.
-
Skin and Eye Contact: Contact with iron(II) oxide powder or solutions can cause irritation to the skin and eyes. Protective gloves and eye protection are recommended.
-
Disposal: Iron(II) oxide should be disposed of properly according to local regulations. Avoid dumping it into waterways or landfills without proper authorization.
Frequently Asked Questions (FAQ)
Q: What is the difference between iron(II) oxide and iron(III) oxide?
A: The key difference lies in the oxidation state of the iron atom. Iron(II) oxide (FeO) contains iron in the +2 oxidation state, while iron(III) oxide (Fe₂O₃) contains iron in the +3 oxidation state. This difference leads to variations in their properties and applications. Iron(III) oxide is more commonly known as rust and is much more stable than iron(II) oxide.
Q: Is iron(II) oxide a naturally occurring compound?
A: Yes, iron(II) oxide occurs naturally in several minerals, though it is less common than iron(III) oxide. It's found in wüstite, a rare mineral typically found in meteorites and volcanic rocks.
Q: Can iron(II) oxide be used as a food additive?
A: While iron(II) oxide is generally considered safe, its use as a food additive is not common. Other forms of iron are typically used for iron fortification in food products.
Q: How is the purity of iron(II) oxide determined?
A: The purity of iron(II) oxide can be determined using various analytical techniques, including chemical titration, atomic absorption spectroscopy, and X-ray diffraction.
Conclusion: The Importance of Iron(II) Oxide
Iron(II) oxide, despite its seemingly simple formula, is a fascinating and crucial chemical compound with a diverse range of applications. Its unique properties, stemming from the +2 oxidation state of iron and its crystal structure, make it valuable in industries ranging from pigments and ceramics to catalysis and metallurgy. Understanding its properties, production methods, and applications is essential for anyone working in related fields. As research continues, we can expect further innovations and advancements in harnessing the potential of this remarkable compound. Its importance in various industrial processes and its potential for future applications solidify its place as a significant chemical entity worthy of continued study and exploration.
Latest Posts
Related Post
Thank you for visiting our website which covers about Formula For Iron 2 Oxide . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.